Description
TribioScience’s Cell Counting Kit-8 (CCK-8) is a colorimetric assay to quantify the viable cells, consisting of the tetrazolium salt and the electron mediator 1-mthoxyPMS. The working principle is based on dehydrogenase activity detection. The generated NADH from the reaction of dehydrogenase enzymes and the substrates oxidizes the 1-methoxyPM. The reduced 1- methoxyPMS further oxidizes the tetrazolium salt (WST-8) in the CCK-8 assay kit, forming the yellow formazan dye. The dye color can then be quantified by measuring the absorbance at 460 nm wavelength so that cell number is known. The assay can be used for the measurement of cell proliferation in response to growth factors, cytokines, mitogens, and nutrients, etc. It can also be used for the analysis of cytotoxic compounds like anticancer drugs and many other toxic agents and pharmaceutical compounds.
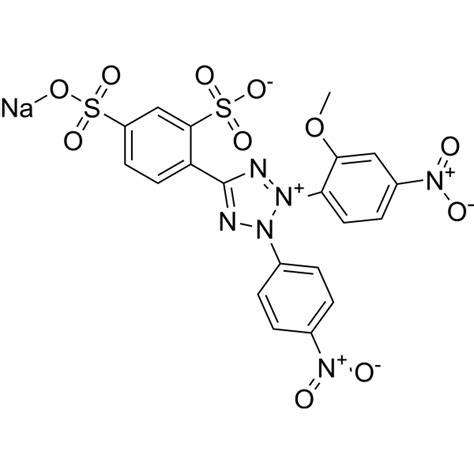
Cell Counting Kit-8 (CCK-8) allows very convenient assays by utilizing Dojindo’s highly water-soluble tetrazolium salt. WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] produces a water-soluble formazan dye upon reduction in the presence of an electron mediator, as shown in Figure 1.
CCK-8 is a one-bottle solution; no premixing of components is required. CCK-8, being nonradioactive, allows sensitive colorimetric assays for the determination of the number of viable cells in cell proliferation and cytotoxicity assays. WST-8 is reduced by dehydrogenases in cells to give an orange colored product (formazan), which is soluble in the tissue culture medium (Figure 2). The amount of the formazan dye generated by dehydrogenases in cells is directly proportional to the number of living cells.
Figure 3 shows that the cell proliferation assay using CCK-8 correlates well with the [3H]-thymidine incorporation assay. Thus, the CCK-8 assay can also be substituted for the [3H]-thymidine incorporation assay. As shown in Figure 4, the detection sensitivity using CCK-8 is higher than assays using other tetrazolium salts such as MTT, XTT, MTS or WST-1.
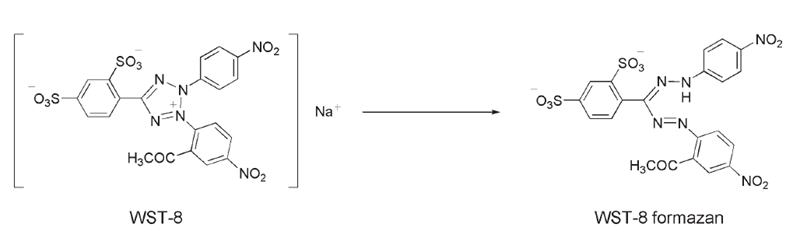
Figure 1. Structures of WST-8 and WST-8 formazan
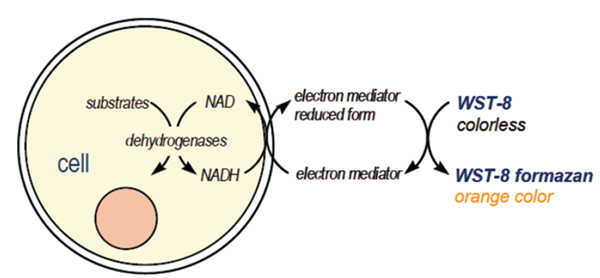
Figure 2. Principle of the cell viability detection with Cell Counting Kit-8
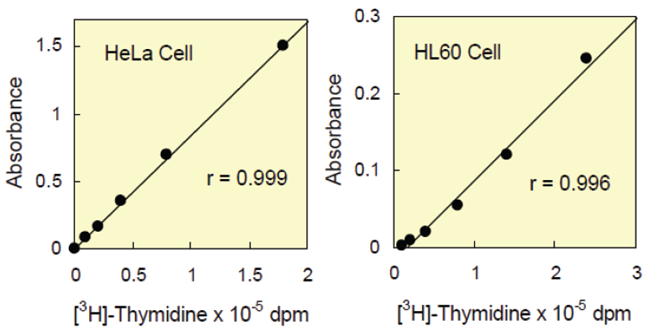
Figure 3 Correlation between CCK-8 assay and [3H]-thymidine incorporation assay.
| Medium : | HeLa…….MEM, 10% FBS |
| HL60…….RPMI1640, 10% FBS | |
| Reagent : | [3H]-Thymidine……..37 KBq/well |
| CCK-8…………………10 μl/well | |
| Incubation : | [3H]-Thymidine assay…4 hours |
| CCK-8……………………3 hours |
Kit Contents
| 100 tests : | 1 ml x 1 |
| 500 tests : | 5 ml x 1 |
| 2500 tests : | 5 ml x 5 |
| 5000 tests : | 5 ml x 10 |
| 10000 tests : | 100 ml x 1 |
Storage
Store at 0-5°C
CCK-8 is stable over one year at 0-5°C with protection from light.
Store it at -20°C for longer storage. Repeated thawing and freezing causes an increase in the background, which interferes with the assay. Please store the kit at 0-5°C for frequent use.
Required Equipment and Materials
- plate reader (450 nm filter)
- 96-well plate
- CO2 incubator
- 10 μl and 100 – 200 μl multi-channel pipettes
General Protocol
Cell Number Determination
- Inoculate cell suspension (100 μl/well) in a 96-well plate. Pre-incubate the plate in a humidified incubator (e.g., at 37 °C, 5% CO2).
- Add 10 μl of the CCK-8 solution to each well of the plate.
- Be careful not to introduce bubbles to the wells, since they interfere with the O.D. reading.
- Incubate the plate for 1 – 4 hours in the incubator.
- Measure the absorbance at 450 nm using a microplate reader.
- To measure the absorbance later, add 10 μl of 1% w/v SDS or 0.1 M HCl to each well, cover the plate and store it with protection from light at room temperature. No absorbance change should be observed for 24 hours.
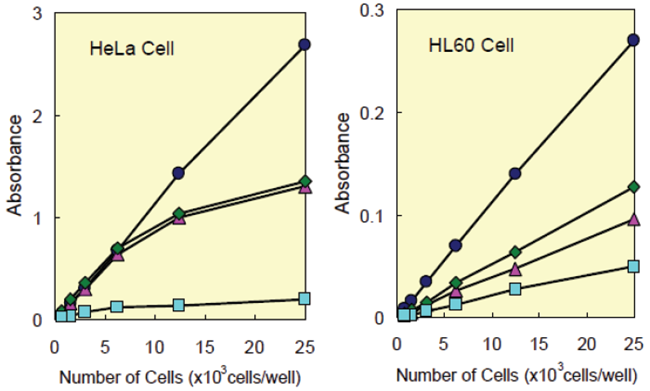
Figure 4 Cell number determination using CCK-8 and other reagents.
| Medium : | HeLa… | MEM, 10% FBS |
| HL60… | RPMI1640, 10% FBS | |
| Incubation : | HeLa… | 37°C, 5% CO2, 2 hours |
| HL60… | 37°C, 5% CO2, 3 hours | |
| Detection : | CCK-8(●)…450 nm, XTT(◆)…450 nm | |
| MTS(▲)…490 nm, MTT(■)…570 nm | ||
Cell Proliferation and Cytotoxicity Assay
- Dispense 100 μl of cell suspension (5000 cells/well) in a 96-well plate. Pre-incubate the plate for 24 hours in a humidified incubator (e.g., at 37°C, 5% CO2).
- Add 10 μl of various concentrations of substances to be tested to the plate.
- Incubate the plate for an appropriate length of time (e.g., 6, 12, 24 or 48 hours) in the incubator.
- Add 10 μl of CCK-8 solution to each well of the plate.
- Be careful not to introduce bubbles to the wells, since they interfere with the O.D. reading.
- Incubate the plate for 1 – 4 hours in the incubator.
- Measure the absorbance at 450 nm using a microplate reader.
- To measure the absorbance later, add 10 μl of 1% w/v SDS or 0.1 M HCl to each well, cover the plate and store it with protection from light at room temperature. No absorbance change should be observed for 24 hours.
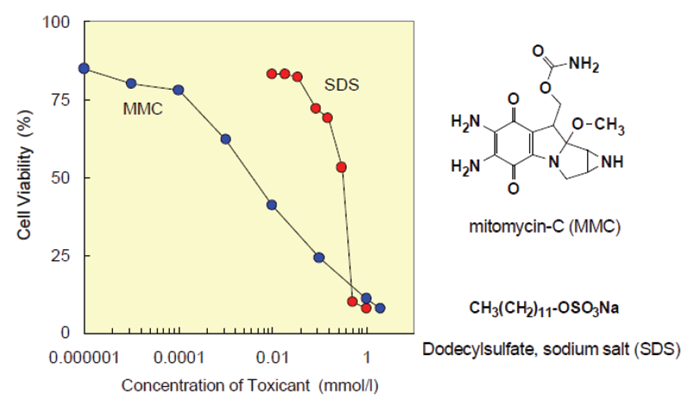
Figure 5. Toxicological test of chemicals using CCK-8.
| Cell line : HeLa Culture medium : MEM, 10% FBS Chemicals : Mitomycin-C (MMC), Dodecylsulfate, sodium salt (SDS) Incubation : 37°C, 5% CO2, 2 hours Detection : 450 nm, Reference : 650 nm |
Precautions
- Since the CCK-8 assay is based on the dehydrogenase activity detection in viable cells, conditions or chemicals that affect dehydrogenase activity in viable cells may cause discrepancy between the actual viable cell number and the cell number determined using the CCK-8 assay.
- WST-8 may react with reducing agents to generate WST-8 formazan. Please check the background O.D. if reducing agents are used in cytotoxicity assays or cell proliferation assays.
- Be careful not to introduce bubbles to the wells, since they interfere with the O.D. reading.
- If the sterilization of the CCK-8 solution is necessary, please filter the solution with a 0.2 μm membrane.
- The incubation time varies by the type and number of cells in a well. Generally, leukocytes give weak coloration, thus a long incubation time (up to 4 hours) or a large number of cells (~105 cells/well) may be necessary.
- Measure and subtract the O.D. at 600 nm or higher from that of sample if there is a high turbidity in the cell suspension.
Frequently Asked Questions
1. How many cells should there be in a well?
For adhesive cells, at least 1000 cells are necessary per well (100 μl medium). For leukocytes, at least 2500 cells are necessary per well (100 μl medium) because of low sensitivity. The recommended maximum number of cells per well for the 96-well plate is 25000. If a 24-well or 6-well plate is used for this assay, please calculate the number of cells per well accordingly, and adjust the volume of the CCK-8 solution in a well to 10% of the total volume.
2. Does CCK-8 stain viable cells?
No. Since WST-8 and its formazan dye are highly water-soluble, CCK-8 cannot be utilized for cell staining purpose.
3. Does phenol red affect the assay?
No. The absorption value of phenol red in a culture medium can be removed by subtracting the absorption value of a blank solution from the absorption value of each well. Therefore, a medium containing phenol red is usable for the CCK-8 assay.
4. Is CCK-8 toxic to cells?
Since the toxicity of CCK-8 is so low, the same cells can be used for other cell proliferation assays such as the crystal violet assay, neutral red assay or DNA fluorometric assay after the CCK-8 assay is completed.
5. I do not have a 450 nm filter. What other filters can I use?
You can use filters with the absorbance between 430 and 490 nm, even though 450 nm filter gives the best sensitivity.
References
- M. Ishiyama, Y. Miyazono, K. Sasamoto, Y. Ohkura and K. Ueno, Talanta, 1997, 44, 1299.
- H. Tominaga, M. Ishiyama, F. Ohseto, K. Sasamoto, T. Hamamoto, K. Suzuki and M. Watanabe, Anal. Commun., 1999, 36, 47.

Reviews
There are no reviews yet.